Origin
Nanofitins® are small, single-chain proteins (7 kDa, around 20 times smaller than a monoclonal antibody). Nanofitins® derive from a naturally hyperstable scaffold, sac7d, from which they retain most of the biochemical features such as resistance to temperature (70-80°C) and pH (0-13).
Proteins from the sac7d family
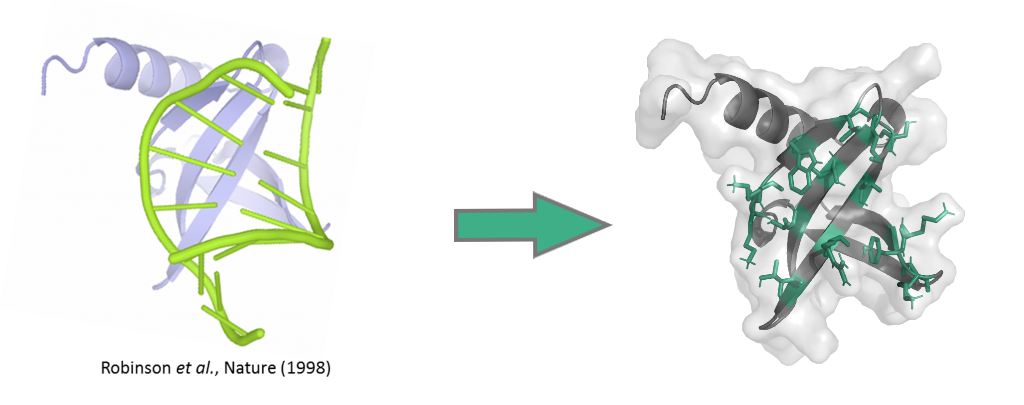
Sac7d is a protein of 66 amino acids (7 kDa) constituted of a single chain folded as an OB-fold (oligonucleotide/oligosaccharide-binding-fold), a β-barrel capped by a C-terminal α-helix, and lacking disulphide bridges. Sac7d belongs to a class of small chromosomal proteins from the hyper thermophilic archaeon Sulfolobus acidocaldarius. This protein, discovered in 1974 in Yellowstone National Park geysers, is extremely stable to heat and acid (natural environment 85°C and pH 2). Often described as a histone-like protein, Sac7d binds to DNA without any particular sequence preference resulting in an increase of the DNA melting temperature by approximately 40 °C, thereby protecting the genome from thermal denaturation.
From natural proteins to efficient ligands
Reengineering of the natural binding site by randomisation of the residue exposed to the solvent (residue in green on figure) allows the creation of libraries of Nanofitin® variants from the wild-type Sac7d protein. Libraries, involving up to 20% of the total residue, are designed so as to fully redirect the molecular recognition against any target presented, with the result that Nanofitins® no longer recognise DNA. Such Nanofitins® combine both a specific, high affinity binding on the target and conservation of the original stability features, i.e. being thermophilic and acidophilic.
Competitive advantages of Nanofitins®
Nanofitins® bear all ligands features to promote affinity chromatography for purification of most biologics: tailored affinity and elution parameters, easy conjugation to resins, low cost-of-goods…

The generation of Nanofitins® consists in identifying the best ligands amongst a vast library of variants with a tunable 100% in vitro selection process. Libraries are screened with many parameters to tune specificity on the target(s): for example to separate a product of interest from its degradation products or monomers from homopolymers. This process is amenable to toxic targets, or non-immunogenic epitopes.
Nanofitins® combine highly specific capture and tunable elution of biologics ranging from small peptides to viruses. Deriving from a naturally hyperstable scaffold, they show resistance to temperature (70-80°C), pH (0-13) and caustic conditions of cleaning-in-place. Identical and regio-selective coupling of the Nanofitins® to a chromatographic support via their conserved region leverages their binding capacity and insures a strong capture of the product to purify. Production of the Nanofitins® in bacterial fermentation ensures a straightforward scale-up, cost-effective manufacturing and batch-to-batch consistency.
Scientific Publications
Affinity chromatography for vaccines purification: EU DiViNe project provides proof of concept
World Vaccines Congress Europe, October 2019
Mikkel Nissum, GSK
Nanofitins® were discovered against GAS25 and the best ligands candidates were characterized and successfully conjugated to resin beads. The affinity columns were tested by GSK in comparison with their current GAS25 purification process, and the affinity chromatography step powered with Nanofitins® replaces 3 chromatographic steps, without compromising on purity of the vaccine since purity is even higher in a single step.
Vaccines purification by affinity chromatography with Nanofitin ligands: demonstration with glycoconjugates
World Vaccines Congress Europe, October 2016
Anne Chevrel, Affilogic
The DiViNe partners are developing a flexible Nanofitin-based platform for vaccines purification and it is illustrated on the poster with the first family of vaccines i.e. the glycoconjugate vaccines. We describe discovery and identification of Nanofitins directed against the CRM197 carrier protein, as well as their conjugation to resin.
Affitins as robust tailored reagents for affinity chromatography purification of antibodies and non-immunoglobulin proteins
(Nanofitins is the commercial name for Affitins)
Béhar G, Renodon-Cornière A, Mouratou B, Pecorari F
J Chromatogr A. 2016 Apr 8;1441:44-51. doi: 10.1016/j.chroma.2016.02.068. Epub 2016 Feb 27
Abstract – Affinity chromatography is a convenient way of purifying proteins, as a high degree of purity can be reached in one step. The use of tags has greatly contributed to the popularity of this technique. However, the addition of tags may not be desirable or possible for the production of biopharmaceuticals. There is thus a need for tailored artificial affinity ligands. We have developed the use of archaeal extremophilic proteins as scaffolds to generate affinity proteins (Affitins). Here, we explored the potential of Affitins as ligand to design affinity columns. Affitins specific for human immunoglobulin G (hIgG), bacterial PulD protein, and chicken egg lysozyme were immobilized on an agarose matrix. The columns obtained were functional and highly selective for their cognate target, even in the presence of exogenous proteins as found in cell culture media, ascites and bacterial lysates, which result in a high degree of purity (∼95%) and recovery (∼100%) in a single step. Anti-hIgG Affitin columns withstand repetitive cycles of purification and cleaning-in-place treatments with 0.25 M NaOH as well as Protein A does. High levels of Affitin productions in Escherichia coli makes it possible to produce these affinity columns at low cost. Our results validate Affitins as a new class of tailored ligands for the affinity chromatography purification of potentially any proteins of interest including biopharmaceuticals.
Affitins for protein purification by affinity magnetic fishing
(Nanofitins is the commercial name for Affitins)
Fernandes CS, Dos Santos R, Ottengy S, Viecinski AC, Béhar G, Mouratou B, Pecorari F, Roque AC
J Chromatogr A. 2016 Jul 29;1457:50-8. doi: 10.1016/j.chroma.2016.06.020. Epub 2016 Jun 7
Affinity chromatography for vaccines manufacturing: Finally ready for prime time?
Zhao M, Vandersluis M, Stout J, Haupts U, Sanders M, Jacquemart R
Vaccine, Volume 37, Issue 36, 23 August 2019, Pages 5491-5503
Extract – Industry, government and funding agencies recognize the need for and feasibility of industrial affinity chromatography strategies for vaccine purification to overcome technical and cost challenges faced in the current manufacturing scheme. DiViNe is a project funded by the European Union involving iBET, Affilogic, Aquaporin, Merck KGaA, GenIbet Biopharmaceuticals, and GSK. The ultimate goal of the project is to develop custom affinity chromatography solutions for vaccine production that can enable affordable and environmental-friendly purification processes and promote access to vaccines in developing countries. A first result of the project was communicated at World Vaccine Congress 2016 demonstrating identification and resin conjugation of customized ligand against CRM197 carrier protein for conjugate vaccines production.
The Future of Protein Scaffolds as Affinity Reagents for Purification
Dias AM, Roque AC
Biotechnology and Bioengineering, March 2017
Extract – The potential for bioseparation will be further explored in purification of vaccines: glycol-conjugates, protein antigens, and viruses, now supported by the European project DiViNe.
Use of the Nanofitin Alternative Scaffold as a GFP-Ready Fusion Tag
Huet S, Gorré H, Perrocheau A, Picot J, Cinier M
PLoS One. 2015;10: e0142304.
Bisphosphonate adaptors for specific protein binding on zirconium phosphonate-based microarrays
Cinier M, Petit M, Williams MN, Fabre RM, Pecorari F, Talham DR, Bujoli B, Tellier C
Bioconjugate Chemistry; 2009 Dec;20(12):2270-7
Artificial binding proteins (Affitins) as probes for conformational changes in secretin PulD
Krehenbrink M, Chami M, Guilvout I, Alzari PM, Pecorari F, Pugsley AP
Journal of Molecular Biology; 2008 Nov 28;383(5):1058-68
Remodeling a DNA-binding protein as a specific in vivo inhibitor of bacterial secretin PulD
Mouratou B, Schaeffer F, Guilvout I, Tello-Manigne D, Pugsley AP, Alzari PM, Pecorari F
Proceedings of the National Academy of Science of the USA. 2007 Nov 13;104(46):17983